

The manufacturing industry uses an array of plastics for the production of plastic parts. Their selection is based upon the chains of carbon molecules as this determines their physical and chemical properties. Their structure affects the strength of the molecules in terms of endurance and durability, the way they interact with the environment, their appearance, and more. In this article, we will help you understand the difference between 2 popular plastic categories – Homopolymers and Copolymers.
In order to understand the difference between homopolymers and copolymers, let’s first understand what polymers are.
Polymers are macromolecules that are formed through the linking of smaller units called monomers. The formation of polymers basically includes the linking of a large number of monomers through chemical reactions. This process of polymer formation through monomer linking is referred to as polymerization.
A polymer’s chemical and physical properties primarily depend upon the type of monomers that are used to form the polymer. Polymers are thus, primarily classified based on the number of different types of monomers that are used to form the particular polymer molecule. There are two types of polymers: homopolymers and copolymers.
The primary difference between homopolymers and copolymers is that homopolymers are produced by using a single type of monomer, whereas copolymers are formed by using two different types of monomers. This difference gives homopolymers and copolymers their unique set of properties.
When a single type of monomer undergoes polymerization to form a macromolecule, it is referred to as a homopolymer. A homopolymer basically has repeating units of a single type of monomer. For example, polystyrene is a homopolymer with repeating units of styrene monomers.
Homopolymers are formed through a polymerization technique that is referred to as ‘addition polymerization’. In this process, monomers must have either a single or double bond.
In most cases, the ‘poly’ in homopolymers is used as a prefix which is then followed by the chemical name of the repeating unit. For e.g. repeating units of ‘vinyl chloride’ monomers forms polyvinyl chloride polymer.
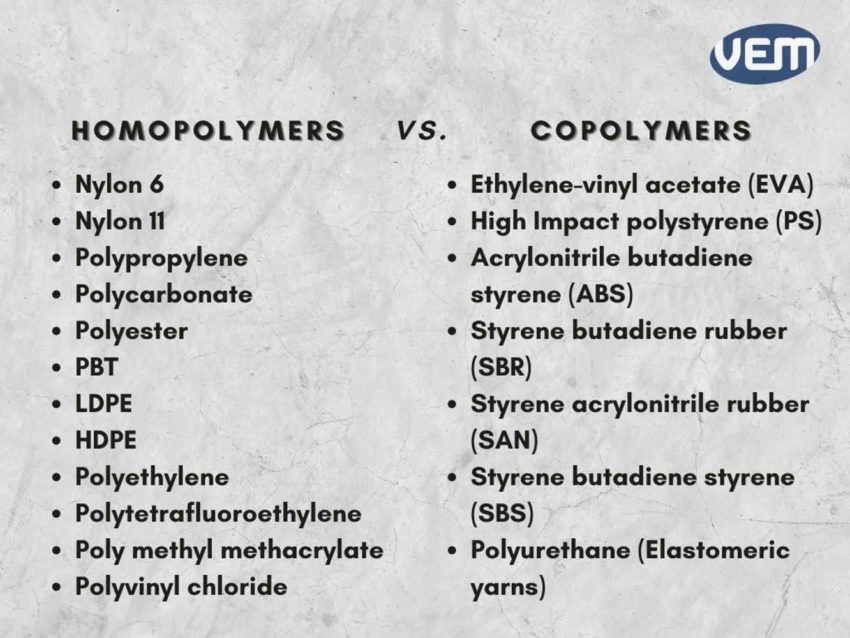
Homopolymer thermoplastics are widely used in injection molding. Some of the most common examples include:
Nylon 6
Nylon 11
Polypropylene
Polycarbonate
Polyester (PET)
PBT
LDPE
HDPE
Polyethylene
Polytetrafluoroethylene
Poly methyl methacrylate
Polyvinyl chloride (PVC)
Polyacrylonitrile
The homopolymers can be applied to various industries. They are also used in different processing technologies, such as injection molding, blow molding, sheet extrusion, and thermoforming.
A polymer that is formed through the polymerization of more than one type of monomer is referred to as a copolymer. Thus, a copolymer always has two or more types of repeating units. Usually, most copolymers are formed through a process called condensation polymerization.
Copolymers are further classified into various categories. As opposed to homopolymers which are a category by themselves, co-polymers are further classified based on the arrangement of the monomers on the main chain.
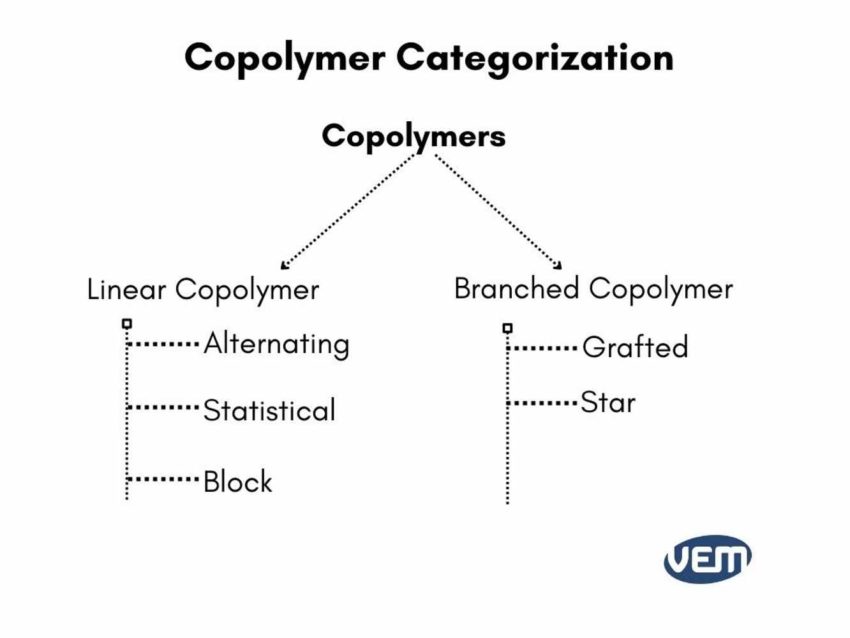
A copolymer has at least two types of monomers, so on the basis of how these monomers are arranged in the chain, the copolymers are further classified into the following basic types:
The linear copolymers contain a single main chain and are sub-classified as either alternating, statistical, or block copolymers. Branched copolymers are further sub-classified into grafted and star-shaped copolymers.
Alternating copolymers include alternating monomers and they have a single main chain. If an alternating copolymer has ‘monomer A’ and ‘monomer B’, then the formula of the alternating copolymer will be (-A-B-)n.
An example of an alternating copolymer is Nylon 6,6 which consists of alternating units of hexamethylene diamine and adipic acid.
When more than one homopolymer unit is linked together via covalent bonds, the resulting single-chain macromolecule is called a block copolymer. The repeating units in block polymers are of the same type. E.g: ̴ ̴ ̴ ̴ ̴A-A-A-A-A-B-B-B-B-B ̴ ̴ ̴ ̴ ̴
An example of a block polymer is styrene butadiene styrene, which is also known as SBS rubber.

Statistical copolymers are polymers in which two or more monomers are arranged in a sequence that follows some type of statistical rule. These polymers are formed through free radical polymerization.
An example of a statistical polymer is Styrene butadiene rubber (SBR) which is a rubber of styrene and butadiene monomers.
If the branched copolymer features differently structured main chains and side chains, it is referred to as graft copolymers. In grafted copolymers, one or more blocks of homopolymer are grafted as branches onto the main chain. Thus, in this type of copolymer, one or more side chains of a homopolymer are attached to the backbone of the main chain.
An example of a grafted polymer is high-impact polystyrene. It consists of a polystyrene backbone with polybutadiene grafted chains.
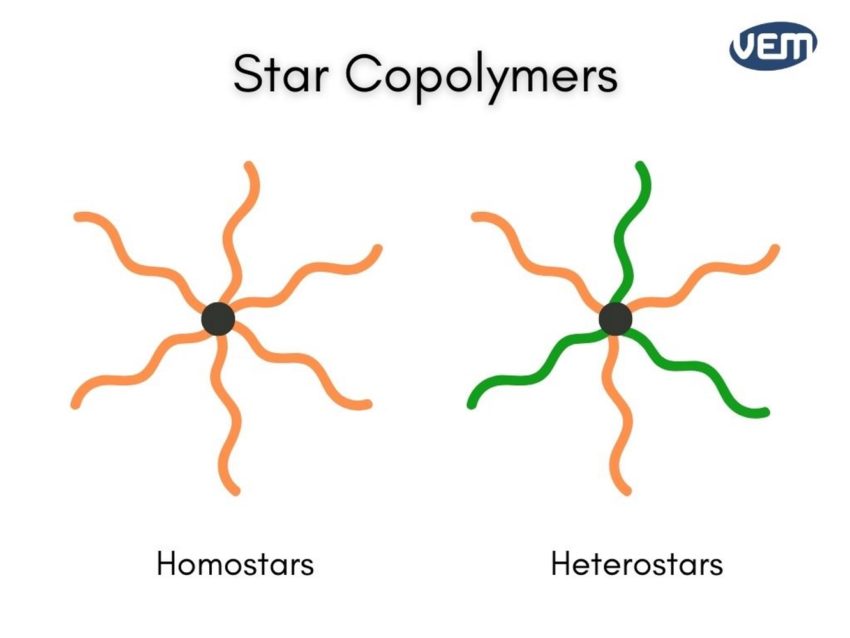
Star-shaped polymers have a multifunctional center. At least three polymer chains (arms) extend through the center. These arms can either be chemically identical which is referred to as homostars or they can be different which is referred to as heteroarm stars.
An example of a star-shaped polymer is polyethylene oxide (PEO).
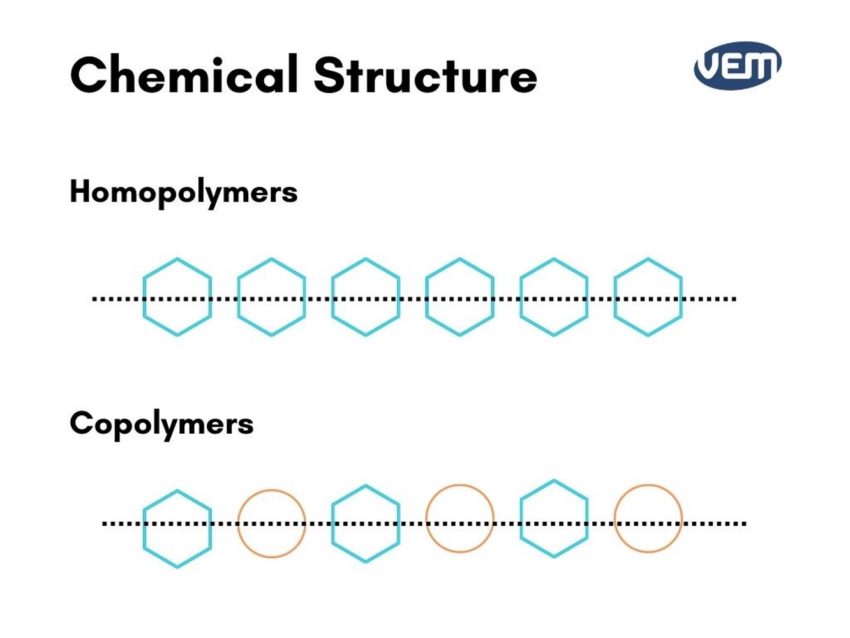
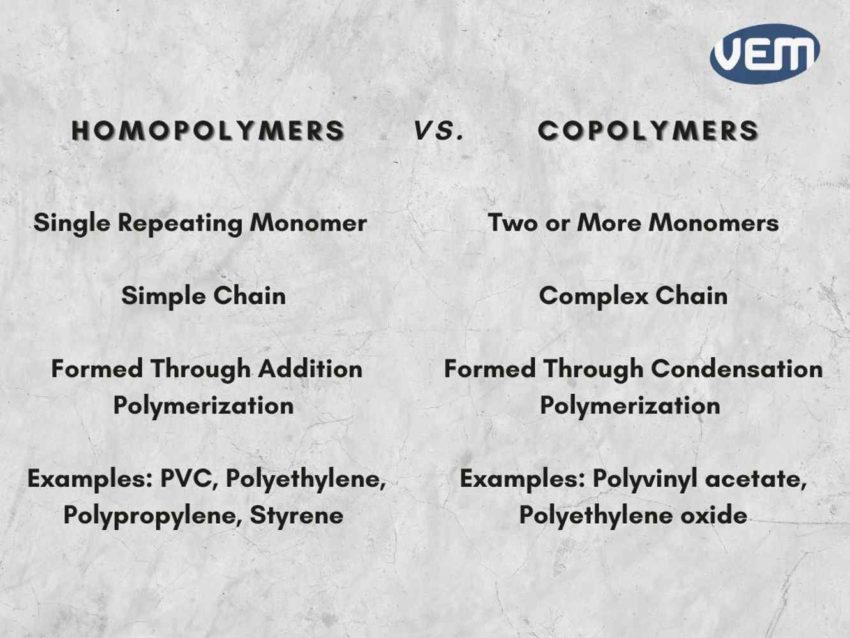
Homopolymers consist of single species of repeating units whereas copolymers consist of two or more types of repeating units.
Homopolymers have a single type of monomer whereas Copolymers have two or more types of monomers.
Homopolymers usually have a simple structure whereas copolymers have a complex structure.
Homopolymers are formed through addition polymerization and copolymers are formed through condensation polymerization.
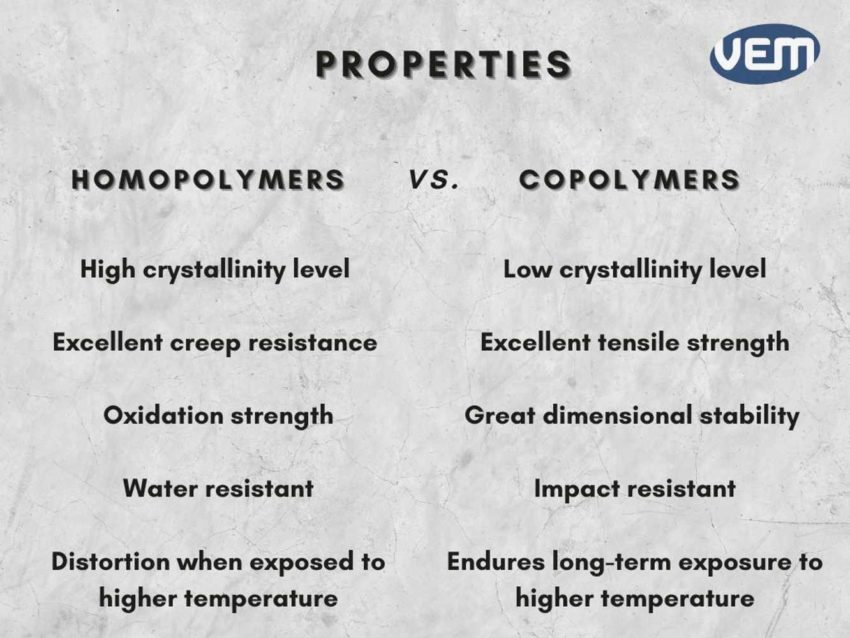
Understanding the difference between homopolymers and copolymers is imperative as it can help you choose the best material for manufacturing your plastic part.
If you have any queries about homopolymers and copolymers, you can reach out to us. Our team has an immense experience of over 20 years with mold making and manufacturing which can surely be helpful for your project.